Philips Recalls Some Sleep Apnea Machines In US Over Toxic Foam Risk
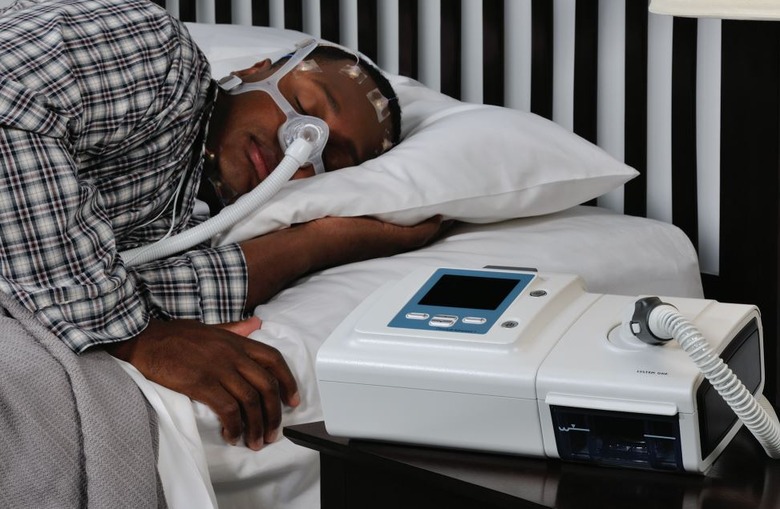
Philips has released the latest details on its sleep apnea machine recall due to concerns about the sound abatement foam used in these devices. The recall impacts consumers in the United States who own certain bi-level positive airway pressure (Bi-Level PAP) and continuous positive airway pressure (CPAP) machines, as well as some mechanical ventilators.
According to Philips, testing has revealed some possible risks associated with the sound abatement foam used in these machines. A key concern is that the foam may release chemicals through a process called off-gassing and the foam may degrade into small particles. The company explains that this degradation may be made worse if the machine is used in environments that have high heat and humidity or if the machines are cleaned using unapproved methods.
Users may end up breathing in these foam particles or chemicals, putting them at various potential health risks. In its medical device recall notification, Philips says exposure to the degraded foam could result in multiple symptoms, including things like headache, irritation, 'adverse effects' on organs, asthma, inflammation, and 'toxic carcinogenic affects,' raising cancer concerns.
Philips says that it has received some reports of potential issues in patients who may have been impacted by the foam degradation issue, but none related to the foam off-gassing risk. As well, there haven't been any reports of death related to this recall. The company is setting up a repair and replacement program as part of this recall that will involve replacing the foam with a different material.
For people who are using one of the recalled bi-level PAP or CPAP machines, Philips says they should stop using the device and contact their medical equipment provider or doctor about an alternative. Patients who don't have an alternative are advised to talk with their doctor about whether the benefits of continuing to use the machine would outweigh the risk associated with the foam.
The impacted devices were made before April 26, 2021, with all of the device serial numbers impacted. The full list of models covered by this recall can be found on the Philips USA website here.
