Moderna's mRNA HIV Vaccine Trial Kicks Off With First Round Of Doses
Moderna has just announced the launch of the first clinical trial for an HIV vaccine made using its messenger RNA (mRNA) technology, and the first doses have already been administered to enrolled participants. The use of mRNA tech is a major milestone in the multi-decade effort to develop an HIV vaccine, arriving decades after the first vaccine clinical trial was conducted by the NIH in 1987.
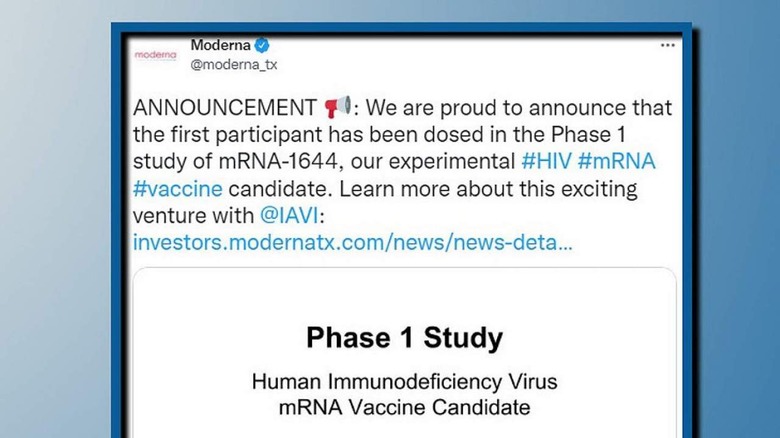
The biotechnology company broke the news in a lengthy blog post, as well as a tweet, on January 27, 2022. The vaccine's development is part of Moderna's collaboration with the International AIDS Vaccine Initiative (IAVI,) a non-profit focused on accelerating the efforts to create a fully accessible HIV vaccine. The Bill & Melinda Gates Foundation is also named as a partner and was responsible for funding the required research.
Phase I of the trial has already begun; the participants received their doses at George Washington University School of Medicine and Health Sciences in Washington, D.C. The vaccine works in a way similar to Moderna's successful COVID-19 vaccine, leveraging messenger RNA to teach the cells to develop and trigger proper immune responses against the virus.
The HIV vaccine is long-awaited and very necessary
Developing an effective vaccine against HIV has been on the radar for nearly four decades, and yet, not a single one of the previously tested vaccines made it past clinical trials. So far, the U.S. Food and Drug Administration (FDA) has not approved any HIV vaccines for use. This means the only way to combat HIV is through medicine.
It's true that these days, HIV can largely be controlled through medication. Once a death sentence, the disease is now entirely manageable to the point where it may not affect lives and is not transmissible — as long as the proper medication is administered, which is not always the case, especially in developing countries.
According to HIV.gov, as of 2020, there were approximately 37.7 million HIV-positive people across the globe. The World Health Organization reports that since the beginning of the epidemic, anywhere between 27.2 and 47.8 million people have died as a result of HIV infections. These numbers alone indicate how crucial it is to find an accessible way to protect people from being infected by the virus in the first place.
As mentioned above, many companies tried to develop an HIV vaccine. Some of these attempts made it as far as Phase III clinical trials. In 1984, Margaret Heckler predicted a vaccine would be developed within two years. Unfortunately, nearly forty years later, all efforts have come up short.
Dr. Fauci expects an impact on the HIV epidemic
The first phase of the trial will involve 56 healthy individuals who are adults and have not been infected with HIV. The research will be carried out at GWU and three other facilities: The Hope Clinic of Emory Vaccine Center in Atlanta, the University of Texas-Health Science Center at San Antonio, and the Fred Hutchinson Cancer Research Center in Seattle.
Moderna has plans for a total of three doses of the mRNA vaccine to be administered, although not all participants will be given all three doses. Forty-eight of the volunteers will be given two doses, out of which 32 will also be given the third booster shot. Eight participants are only going to receive the booster shot and no other vaccine doses. For six months after their final dose, the participants will be closely monitored for side effects and to assess their immune responses to the vaccine.
For decades, experts have made it clear that vaccination is one of the crucial steps towards controlling the spread of HIV and the development of AIDS. "It goes exactly to what I have been saying for years and years — if you implement the tools that you have, you will definitely see an impact on the dynamics of the epidemic," Dr. Anthony Fauci said in a 2019 interview with NBC News about HIV vaccine trials and medication.