Metallic Hydrogen Finally Created, Could Change Life As We Know It
You might soon have to change what you think you know about hydrogen. The simplest and lightest element and commonly regarded as a gas, hydrogen can actually take on liquid form when cooled. And now it can also take on a metal form as well. Yes, atomic metallic hydrogen has just been made real, delivering one of the holy grails of physics. And before you brush it off as just some geeky achievement, metallic hydrogen has the potential to revolutionize technology and life forever.
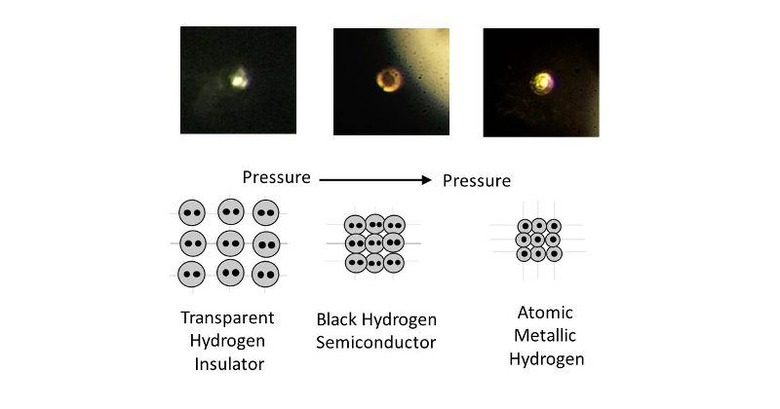
It has been decades, nearly a century, since the possibility of a metallic form of hydrogen has been theorized. But it is only now that science, technology, and mechanics have finally caught up with the theory. In principle, the creation of metallic hydrogen isn't that different from how a diamond is formed, which involves inordinate amounts of pressure. In the case of hydrogen, it required 495 gigapascals, roughly more than 71.7 million lbs. per inch, of pressure. That's greater than the pressure at the center of the earth.
This results in the super compact, "meta-stable" superconductor that is known as atomic metallic hydrogen. Being meta-stable means that it won't revert into its previous liquid then gas state when the pressure is removed, pretty much like how a diamond stays a diamond even without pressure. It's the semiconductor part that has scientists giddy about the possible applications of this new but still rare material.
The problem with most, if not all, conductors is that they lose energy, as much as 15%, due to the dissipation of heat. Metallic hydrogen, however, doesn't have such a problem and can conduct electricity even at room temperatures, which will revolutionize how we transmit and store energy. Even beyond that superconducting application, metallic hydrogen could also benefit space science. Because of how much energy is used to turn molecular hydrogen into metallic hydrogen, reversing the process will release that same amount of energy, which could be harnessed for propulsion systems.
Of course, it's too early to pin this down as a complete victory, because we'll need to be able to produce mass amounts of metallic hydrogen cheaply for any of that to become reality. Still, after almost 80 years of trying to actually make metallic hydrogen, there's enough reason to break out the champagne.
SOURCE: Phys.org