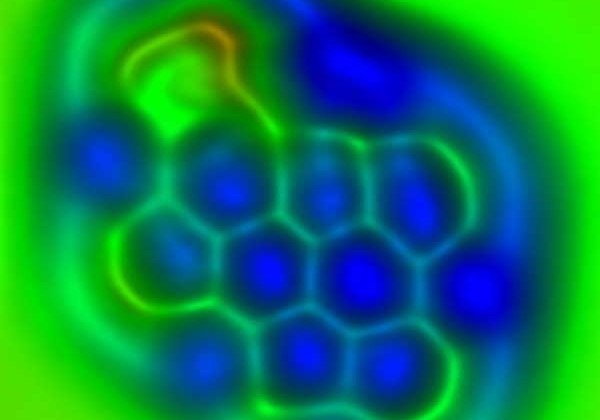
IBM Scientists First To Differentiate The Chemical Bonds In Individual Molecules Using AFM
IBM has announced that its scientists have been able to differentiate the chemical bonds in individual molecules for the first time via a technique called non-contact atomic force microscopy (AFM). The breakthrough has significant implications for the technology world. According to IBM, the breakthrough will help push the exploration of using molecules and atoms at a smaller scale and can be an important step for studying graphene devices.
Graphene devices are being studied as potential replacements for existing technologies used for microchips. Graphene is predicted to eventually have applications in high-bandwidth wireless communications and electronic displays. Researchers at IBM have been able to image the bond order and length of individual carbon-carbon bonds in C60. C60 is also known as a buckyball thanks to its football shape and to planar polycyclic aromatic hydrocarbons resembling small flakes of graphene.
IBM notes that individual bonds between carbon atoms in these molecules differ slightly and subtly in length and strength. Those subtle differences in the length and strength of bonds between carbon atoms are responsible for the important chemical, electronic, and optical properties of such molecules. IBM's breakthrough marks the first time the differences in those individual bonds were detected in both individual molecules and individual bonds.
The IBM scientist's discovery also shines light on potential new areas of research, including study of the relaxation of bonds around defects in graphene and the changing of bonds in chemical reactions and in excited states. The IBM scientists used an atomic force microscope with a tip that ended with a single carbon monoxide molecule. The tip of that atomic force microscope oscillates with an amplitude above the sample to measure the forces between the tip and sample such as a molecule, and creates an image. The technique made it possible to distinguish individual bonds that differ by only three picometers, which is one-hundredth of an atom's diameter.