FDA Says Coronavirus Vaccine Could Take 1 Year, Despite Trump's Malaria Drug Claims
A vaccine trial currently being performed by the FDA is expected to take "12 months" according to FDA Commissioner Stephen Hahn. This came right after President Donald Trump suggested that the drug hydroxychloroquine (HCQ) showed "very encouraging results" with COVID-19, and that "we're going to be able to make that drug available almost immediately."
"That's where the FDA's been so great," said Trump. "They've gone through the approval process, it's been approved. They took it down from many months... to immediate. So we're going to be able to make that drug available by prescription."* Despite what Trump claimed, FDA Commissioner Hahn suggested that the "immediate" part wasn't entirely true.
*You cannot go get a bottle of chloroquine at your local CVS. This is not like buying a bottle of Tylenol. Not because it's scarce, but because it's not something that's "available immediately" for everyone. It's something you'd get if you had malaria. It's not been tested in doses that'd necessarily fight COVID-19.
"That's a drug that the President has directed us to take a closer look at as to whether an expanded use approach to that could be done and to actually see if that benefits patients," said Hahn. "And again, we want to do that in the setting of a clinical trial, a large, pragmatic clinical trial to actually gather that information."
On how long it'd actually take to get a COVID-19 vaccine, Hahn said several things. "Over the next couple of weeks, we'll have more information that we're really pushing hard to try to accelerate," said Hahn. "That will be a bridge to other therapies that will take us three to six months to develop."
"This is a continuous process," said Hahn, "there is no beginning and end." Hahn added that a vaccine trial was currently being performed (not necessarily with this HCQ drug) that is currently expected to take "12 months." As noted by the authors of the study published on HCQ testing with COVID-19, this drug cannot be used to cure COVID-19 right now, as no clinical tests have yet been run.**
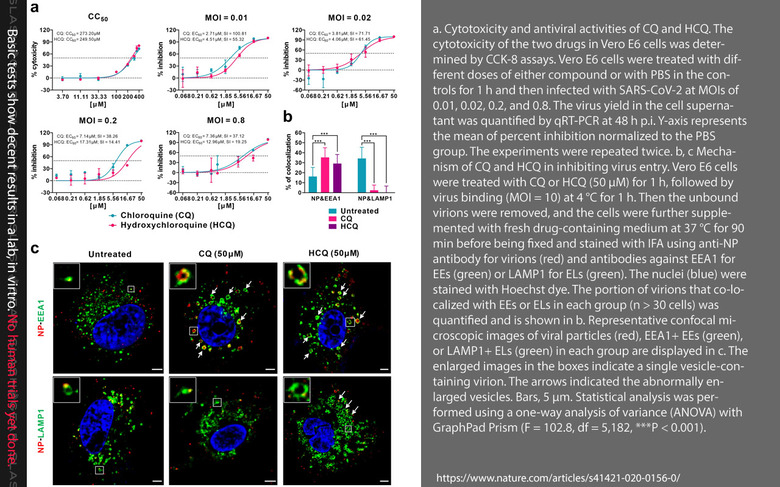
Information on the malaria drug was shown in a study published this week, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, appeared in the publication Nature (Cell Discovery). This study was authored by Jia Liu, Ruiyuan Cao, Mingyue Xu, Xi Wang, Huanyu Zhang, Hengrui Hu, Yufeng Li, Zhihong Hu, Wu Zhong, and Manli Wang. This research can be found with code DOI:10.1038/s41421-020-0156-0 as received on February 24, 2020, and accepted on March 4, 2020.
**This study ran no clinical trials with human patients with the HCQ malaria drug. That study showed HCQ to "efficiently inhibit SARS-CoV-2 infection in vitro." The study's authors made special note of the fact that HCQ is toxic, and "prolonged and overdose usage can still cause poisoning." They concluded, saying "the relatively low SI of HCQ requires careful designing and conducting of clinical trials to achieve efficient and safe control of the SARS-CoV-2 infection."
NOTE: COVID-19 is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). For more information on COVID-19, see: Coronavirus COVID-19 resources: What should I do? Who do I trust?
