FDA Warns This Rapid COVID-19 Test Is Giving False-Negatives
One of the coronavirus tests currently in use in the US to track COVID-19 cases may be giving more false-negative results than expected, the FDA has warned, raising the possibility that infected people may be unwittingly told that they do not have the disease. The US Food and Drug Administration warned that Abbott's ID NOW test could be inaccurate, and giving those with COVID-19 a false sense of security.
The Abbott ID NOW point-of-care test was granted authorization to go into use back in February, as part of the FDA's EUA program. The Emergency Use Authorization system is designed to grant early access to new treatments and diagnostics, in the absence of an approved vaccine, test, or treatment for dealing with COVID-19.
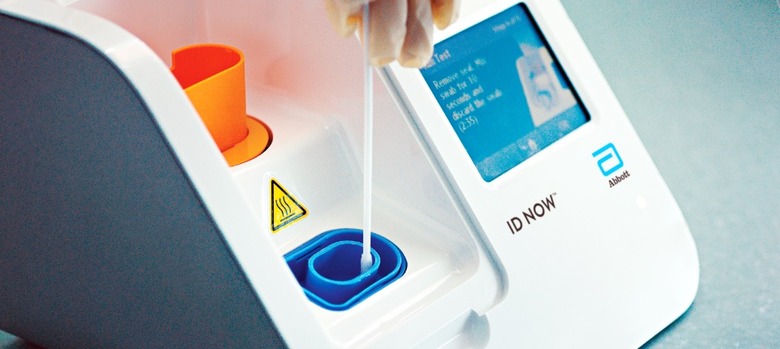
Abbott got that authorization on March 27, for a molecular test which uses the company's ID NOW molecular platform machine. Part of the reason it's appealing is that it's much faster than other coronavirus tests, giving results in 13 minutes or less, rather than the hours that many others require. Over time, though, Abbott has modified the instructions on how best to use the system for COVID-19 diagnostics.
In early May, for example, it warned hospital and academic labs not to use a so-called viral transport media with the machine. That's a liquid used to store, preserve, and transport a virus sample: basically, the swab that took the nasal sample was placed into a vial of transport liquid, which was in turn put into the ID NOW. That, Abbott said, wasn't how the system was meant to be used.
"What we've learned is that this method can reduce the sensitivity of the test through dilution," Norman Moore, Ph.D., director of Scientific Affairs for Infectious Diseases, Rapid Diagnostics at Abbott, explained in a statement at the time, "which could potentially lead to false negative results." Instead, the sample swab should be applied directly to the machine.
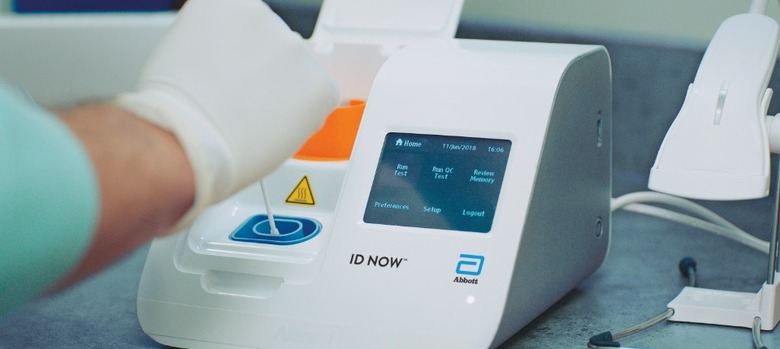
This week, the FDA issued an alert "in the spirit of transparency" about Abbott's system. While no diagnostic is expected to be 100-percent accurate, there have been 15 false-negatives reported to the FDA that the agency is now reviewing.
"We are still evaluating the information about inaccurate results and are in direct communications with Abbott about this important issue," Tim Stenzel, M.D., Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health in the FDA's Center for Devices and Radiological Health, said this week. "We will continue to study the data available and are working with the company to create additional mechanisms for studying the test. This test can still be used and can correctly identify many positive cases in minutes. Negative results may need to be confirmed with a high-sensitivity authorized molecular test."
Abbott, meanwhile, issued its own statement about the testing system. "We're seeing studies being conducted to understand the role of ID NOW in ways that it was not designed to be used," the company argued. "In particular, the NYU study results are not consistent with other studies. While we've seen a few studies with sensitivity performance percentages in the 80s, we've also seen other studies with sensitivity at or above 90%, and one as high as 94%."
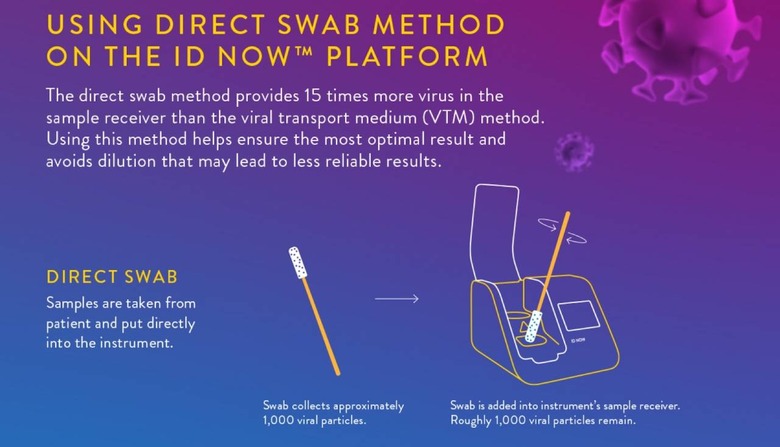
It's unclear at this stage whether the diagnostic failures are down to how the samples were taken, stored, or tested, or if it could be an issue related to the direct swab test method versus viral transport media being used. Abbott will be undertaking studies on ID NOW that will each include at least 150 COVID-19 positive patients in a range of clinical settings, which it's hoped will help get to the bottom of the results disparity.
Altogether, though, it's a reminder that even diagnostics in wide use currently are still being applied without full FDA approval. Testing during the coronavirus pandemic remains controversial, with experts arguing that the US is falling far short in the number of diagnostics being applied in order to get an accurate view of the spread of COVID-19. As of May 14, the CDC says that there are 1,384,930 cases of the disease in the US, and that it has been responsible for 83,947 deaths, though some virologists believe those figures wildly underestimate the true extent of the situation.
