Deep Learning AI Diagnosed Alzheimer's 6-Years Earlier Than Conventional Methods
One of the most important things for combating Alzheimer's disease is early diagnosis so treatments for the condition can start before damage is severe. The earlier interventions start, the better the outcome for the person suffering from the condition. A new study was published to the medical journal Radiology has found that early prediction for Alzheimer's disease later in life can be made using PET brain scans and AI technology.
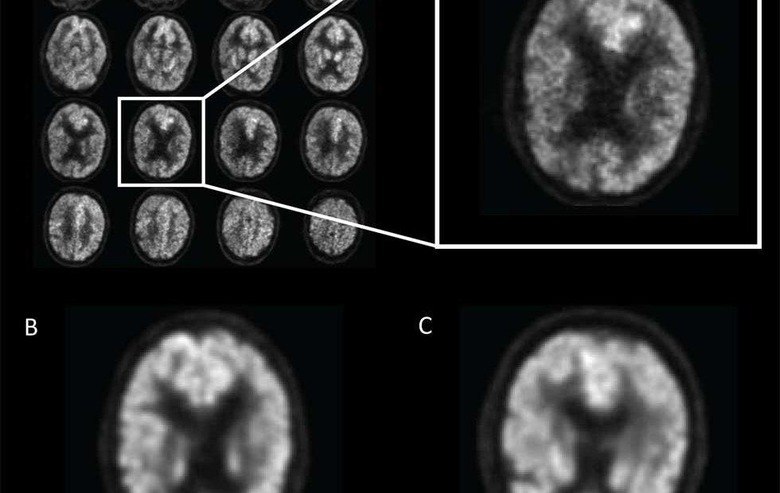
Research study co-author Jae Ho Sohn, M.D. was approached by the senior author of the study, Benjamin Franc, M.D., with interest in applying deep learning AI to find metabolic changes in the brain that are predictors of Alzheimer's disease. The team then trained the deep learning algorithm using imaging technology known as 18-F-fluorodeoxyglucose positron emission tomography (FDG-PET). FDG is a radioactive glucose compound that can be injected into the blood, and the scan can then measure the uptake of FDG in the brain cells; that uptake is an indicator of metabolic activity in the brain.
The researchers had access to over 2,100 FDG-PET brain images from 1,002 patients and trained the deep learning algorithm on 90% of that dataset; the remaining 10% of the dataset was used to test the AI. Deep learning allowed the algorithm to teach itself metabolic patterns that correspond to Alzheimer's disease. After training the algorithm, the scientists tested it on a new set of 40 imaging exams from 40 patients that it had never studied.
In that test, the algorithm was able to score 100% at detecting the disease on an average of 6 years earlier than the final diagnosis was made. Dr. Sohn says that the algorithm the team created and trained was able to predict every single case that advanced to Alzheimer's disease. The team does note that this test was small, and the algorithm needs further validation with a multi-institutional study.
The significant benefit with this new AI is that diagnosing Alzheimer's early can allow treatments and interventions before the loss of brain volume is so massive that intervention is too late. In the future, the team wants to train the algorithm to look for other patterns associated with accumulation of beta-amyloid and tau proteins, both markers specific to Alzheimer's disease.