Coronavirus Blood Filtration System Gets FDA Nod For Emergency Use
The FDA has given emergency authorization to a blood purification system for coronavirus treatment, that promises to filter out potentially lethal proteins in a patient's bloodstream that can surge during COVID-19 infection. The system aims to avoid what's known as a "cytokine storm," where a build-up can lead to rapidly progressive shock, respiratory and organ failure, and even death.
Cytokines are a category of small proteins that the body uses for cell signally. Typically, they mediate and regulate things like immunity and inflammation, working like chemical messengers by binding to the surface of a cell.
In advanced COVID-19 cases, however, cytokines and other inflammatory mediators can rapidly increase in number. That can create inflammation, the immune system becoming hyper-activated as a flood of the chemical messengers becomes uncontrolled. The subsequent hyper-inflammation in the respiratory system can lead to complete failure, and the patient's death.
A blood purification system can filter out the chemical messengers
One way of treating that excess is to use filtration. In effect, the blood is stripped of the extra cytokines, and then returned to the patient's body.
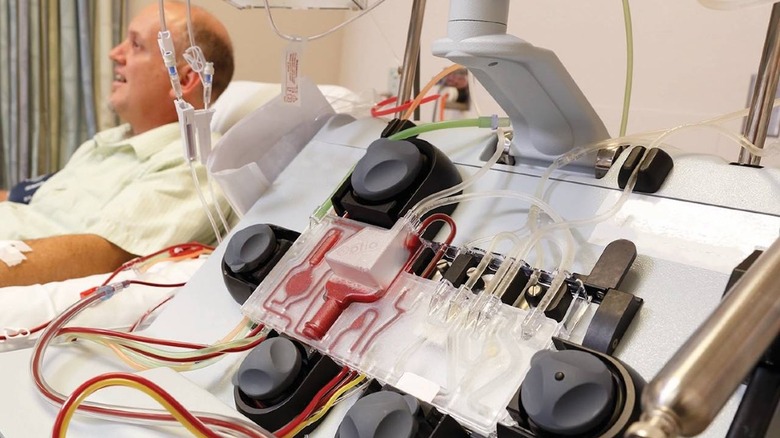
To do that you need a special purifier, also known as an extracorporeal blood purification (EBP) device. One such system has been granted an Emergency Use Authorization (EUA) under the FDA's push to roll out potentially helpful medical technologies during the COVID-19 pandemic. It comprises a machine called the Spectra Optia Apheresis System, combined with a Depuro D2000 Adsorption Cartridge.
First, the Spectra machine is used to separate plasma from whole blood from the patient; less than a cup of blood is typically within the machine's tubing at any point. That plasma is then passed through the Depuro filer, which removes pro-inflammatory cytokines. Finally, it's returned to the patient. The following video demonstrates how such a system could be used in the treatment of sickle cell disease:
It's not a totally risk-free approach, however. The FDA warns that the Term and Depuro devices can lead to very low blood pressure and even reduced delivery of blood to vital organs, and to an abnormal heart rhythm. Other potential risks include bleeding, clotting, infection, and either damage to, or a reduction in, blood cells. It's also possible that a stroke could be triggered, should air get into the bloodstream.
Only a select few COVID-19 patients will get blood purification
While the hunt is on for a successful coronavirus treatment, this filtration system isn't going to be suitable for every patient infected with the virus. According to the FDA, the rule of thumb will be evidence of confirmed or imminent failure of the respiratory system.
It'll only be allowed for "treatment of patients with confirmed COVID-19 who are showing certain severe symptoms (including high fever, persistent cough, developing more difficulty breathing) indicating that they are likely to get more ill within the next 1-24 hours," the US Food and Drug Administration cautions. "These devices have the potential to remove substances in your blood that are not allowing the immune system to function normally."
The EUA lasts for the duration of the coronavirus pandemic, and after that point the two devices will no longer be approved for use in these circumstances.