At-Home Saliva COVID-19 Test Gets FDA Nod: Here's How It Works
The first at-home COVID-19 test that uses saliva samples has been given authorization to launch, the US FDA confirmed today, adding a potentially more convenient and less uncomfortable way to be diagnosed with coronavirus. Though the U.S. Food and Drug Administration has given the green-light to more than 80 COVID-19 tests so far under its emergency clearance program, the majority are designed for healthcare professionals to administer.
That presents a problem when healthcare facilities are overwhelmed with numbers of patents infected with the virus. It also represents a risk, given any person coming in to be tested stands a chance of potentially infecting other people present, or of being infected by them.
The nature of the most common test procedure also has its challenges. Nasal swabs are the most common type of sampling process, but they require a deep swab to reach the right areas. That's not only uncomfortable, but it also triggers a sneeze response that demands healthcare workers administering the test must wear – and repeatedly replace – their personal protective equipment (PPE).
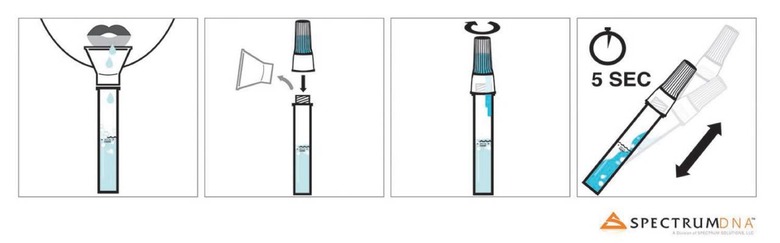
This saliva-based test, for Rutgers Clinical Genomics Laboratory's test, uses a different method. It relies on the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device, into which the person being tested spits. A lid is screwed on, and the vial shaken for five seconds. At that point it can be shipped back to Rutgers for the actual testing process. According to the FDA, the test will remain prescription-only.
Authorization for the use of the test and the at-home saliva sampling comes under the FDA's emergency use authorization (EUA) process. That was implemented at the start of the coronavirus pandemic, as a way to fast-track potential treatments, tests, and more into use, given there is no current treatment or vaccine for COVID-19.

It's not the first at-home test, mind. That, the Laboratory Corporation of America (LabCorp)'s COVID-19 RT-PCR Test, was authorized in April. It uses nasal swabs, though only from the inner edge of the nose and no deeper. Like the Rutger test, it needs a doctor's order in order to be used, and it still requires processing at the company's own laboratories rather than giving a diagnosis at home in the manner of, say, a pregnancy test.
For now, that's not something being offered, though it's definitely being worked on. A team of researchers announced a new CRISPR-based method of testing in April, which is claimed to make at-home testing possible with an almost instant result.
