This Company Plans To Bring The Woolly Mammoth Back From Extinction: Here's How
A few decades ago, Michael Crichton and Steven Spielberg brought the concept of de-extinction to the big screen in "Jurassic Park." Now, the idea has jumped off the screen and into reality, courtesy of Colossal Biosciences, billed as the world's first and only de-extinction company. Instead of dinosaurs, Colossal has set its sights on more recently lost species including the dodo, the thylacine — also known as the Tasmanian tiger– and the woolly mammoth.
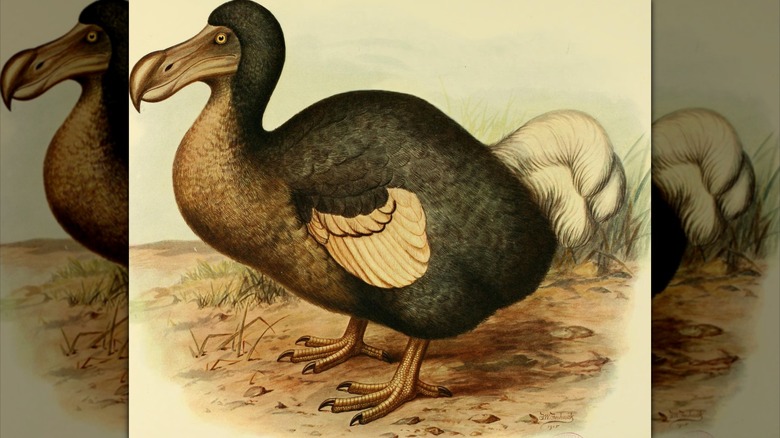
The last confirmed thylacine died in 1936, which is recent enough that you can find photographs of living Tasmanian tigers online. The dodo vanished from the island of Mauritius, which lies to the east of Madagascar, in the late 1600s just decades after humans first arrived on the island. Most of the mammoths died out about 10,000 years ago but isolated populations remained in Siberia and Alaska until about 4,000 years ago. On evolutionary timescales they disappeared only a moment ago, but bringing them back is no easy feat.
"We have animal operations, embryology, genetic engineering, computational biology, all these different groups. We really wanted to parallel path everything. If we did this in a linear fashion it would take significantly longer. We've taken a software approach to this in trying to systematize it, building out an end-to-end process but not doing it in a linear fashion. While we're doing computational biology, we're also advancing the cellular engineering edits, we're also working on impact to elephant conservation, and embryology in elephants," Colossal's CEO Ben Lamm told SlashGear.
What de-extinction means (to Colossal)
When we think about de-extinction, we tend to imagine recovering a species exactly as it was thousands or millions of years ago, but that's not how it's likely to work. After all, even the fictional scientists in "Jurassic Park" had to use DNA from frogs and other modern animals to fill in the genetic gaps. Whether in fiction or reality, it's not really possible to achieve biological and historical truth.
"That's in part because of technological challenges, but also because an organism is more than just the sequence of its genome," said Dr. Beth Shapiro, Colossal's Chief Science Officer. "It's a combination of the DNA code and the environment in which it lives and develops. Of course, extinct environments don't exist anymore."
For its purposes, Colossal defines de-extinction as the creation of an animal which is aesthetically and functionally the same as its predecessor, capable of filling its prior niche, while being better adapted to the world as it is today, not as it was in the past.
"If our goal is ultimately to create species that we're able to put back into natural habitats and restore missing ecological interactions, then we don't need something that is 100% identical to a mammoth and adapted to the ice age, "Shapiro said. "We see de-extinction as the resurrection of core traits, of core ecological interactions... and in some cases even augmenting those traits to help these animals persist in present day habitats."
Preservation of living threatened species
While projects like the resurrection of the mammoth, dodo, and thylacine are attention grabbing, de-extinction isn't Colossal's sole focus. In the course of its de-extinction work, the company is developing new tools, technologies, and strategies with conservation applications. The idea is that any tool which can help to resurrect an extinct species can probably help to conserve an endangered but still living one.
"We know that conservation works, it just doesn't work at the speed at which we are changing the planet. The northern quoll is a great example. If the northern quoll had enough time, they could develop a cane toad toxin resistance, just like other mammals in South America did. But because we introduced the species, and because they don't have the time and population numbers to evolve, they will go extinct," Lamm said. "One genetic change makes them resistant to all cane toad toxins, which is amazing because they love eating cane toads."
Meanwhile, Colossal is working to help other amphibians in other parts of the world. The Colossal Foundation, the charitable arm of the company, recently announced a $3 million donation to combat chytrid, a fungus which has caused amphibian extinctions all over the world. That's in addition to partnering with groups like BioRescue to preserve the functionally extinct northern white rhino. "We're seeing real-world applications to species preservation today, while we're bringing back the species of yesterday," Lamm said.
Biobanking
While Colossal is focused on resurrecting species which were lost hundreds or thousands of years ago, the company is already thinking about species which might go extinct in the near future. To that end, Colossal is working on biobanking strategies as an important part of an ongoing conservation strategy.
Biobanking involves the preservation of genetic material and information. It's achieved by storing physical backups in the form of tissue samples in liquid nitrogen and digital backups in the form of genome sequencing data stored and shared all around the world. As species continue to be threatened and go extinct, biobanking efforts could make future de-extinction projects easier to achieve.
Researchers can go out into the field, collect tissue samples from living animals, take those samples back to the lab, culture and grow the cells, and freeze them in liquid nitrogen. If that species ever goes extinct or they reach a population bottleneck and lose some of their genetic diversity, scientists could use those stored cells to restore that diversity or bring those species back. Likewise, by sequencing genomes not just from a wide range of species but also from as many individuals as possible within that species, we can have a digital frame of reference to know which traits have been lost.
The mammoth, the thylacine, the dodo, and more
Colossal first made headlines a few years back after setting a bold goal to deliver the first woolly mammoth by 2028. Since then, Colossal has added the thylacine and the dodo to its list of projects, in addition to a growing list of threatened species.
"These species are iconic. When people first learn that we can't bring back dinosaurs, the first thing they turn to is a mammoth," Shapiro said. "Every species that's a candidate for de-extinction has a different suite of technical, ethical, and ecological challenges that we need to be able to address in order to do this."
In addition to being iconic, Colossal chose those three species because they are different enough (a placental mammal, a marsupial mammal, and a bird) to represent a large subset of vertebrate biology. Each animal group will require its own tools and processes, the development of which will have wider applications.
"This means that as we move along that path toward bringing those back to life, we have opportunities to develop technologies that are immediately applicable across this huge diversity of life to help these species be able to adapt to their changing habitats and changing role. It gives us an opportunity to develop a suite of tools and resources that have really broad conservation applications as well as learn a lot about basic evolutionary biology." Shapiro said.
Frozen mammoths and other preserved samples
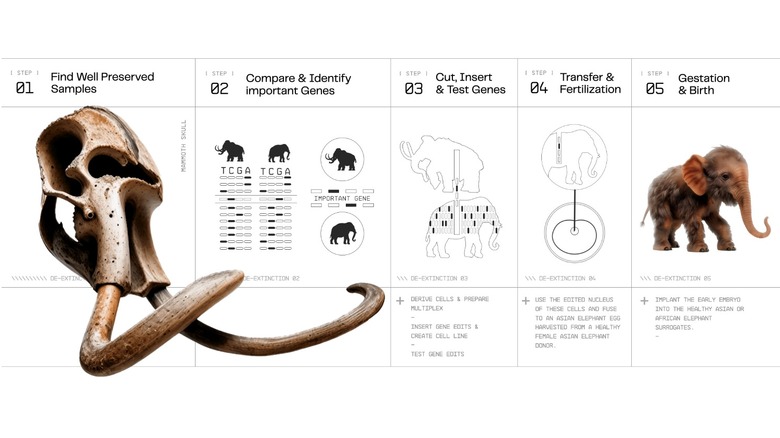
Before you can make a mammoth — or anything else — you need to know the recipe. Genetic work begins with the collection of physical samples which can be studied and analyzed. In the case of the dodo or the Tasmanian tiger, there are museum specimens which are recent enough to provide at least some useful DNA. The mammoth is considerably older, but some of them died inside the planet's deep freezer.
Unlike many extinct species, the mammoth has been remarkably preserved in perpetually cold places like the Siberian tundra. Animals who died tens of thousands of years ago have been recovered in recent years, having decayed almost not at all. For instance, in 2022, miners in the Yukon cut into a chunk of the permafrost looking for gold — but they found a baby mammoth instead.
That tiny mammoth died approximately 30,000 years ago but was so well preserved it looked as if it might have died just days before. These remains and others like them offer uniquely preserved samples for scientists to work with. While the last mammoths disappeared 4,000 years ago and most died out long before that, their DNA remains hidden in the ice.
Genome sequencing
Preserved samples contain crucial genetic information, the sequence of genes which makes a mammoth a mammoth. The genome is a recipe, a list of genetic ingredients which tells scientists what gave mammoths their trademark characteristics including thick fur, curling tusks, and incredible stature.
Of course, if you want to understand the mammoth at a species level, it isn't enough to sequence the DNA of just a single individual. Instead, scientists want to sequence the genome of as many mammoths as possible. One reason is that the DNA of an individual animal may be decayed or otherwise incomplete, even if that animal has been preserved in the permafrost.
Sequencing the DNA of multiple individuals allows scientists to fill in the gaps in the sequence and create a mammoth recipe which is as complete as possible. Even if the DNA of an individual is complete, one mammoth's genome is not the entire mammoth genome. The goal isn't to create a single individual to put in a zoo somewhere, but to create a robust population which could be reintroduced to the wild. The species will have had a range of genetic diversity that can't be captured from a single sample, and we'll need that diversity to create a healthy population of mammoths.
Sequence closely related species
In addition to studying and sequencing the target species, scientists are also focused on studying their closest living relatives. In the case of the thylacine that's the fat-tailed dunnart, in the case of the dodo it's the Nicobar pigeon, and in the case of the mammoth that's every living elephant species. The combined data of the target species and its close genetic relatives helps to provide a clearer picture of the whole genome. Understanding the genomes of living relatives provides a couple of key benefits in the quest for de-extinction.
First, it gives scientists a foundational genome to work from. The Asian elephant, for instance, is the living animal with the fewest number of genetic differences from the mammoth. You can use it as a sort of master recipe from which to build. There's no need to build a mammoth from scratch when you can just make a modified Asian elephant instead.
The second benefit comes back to understanding genetic diversity. Mammoths are just one member of a larger animal group which includes living elephants, mammoths, mastodons, and more. By understanding the genetic diversity of living elephants and extinct relatives, scientists can develop a better understanding of the sorts of diversity which mammoths might have lost, then figure out ways to restore it.
Identify important genes
Colossal doesn't intend to craft a perfect recreation of extinct species. It's not really important to get every single letter of the sequence in exactly the right place. Instead, scientists will target specific genes associated with desired traits. In the case of the mammoth, those are the genes for ear size, fur density, cold adaptation, and a host of other qualities which make mammoths distinct from their living relatives.
"We have these alignments of mammoth genomes and alignments of elephant genomes and we compare them to each other to find out where all the mammoths are the same as each other and different from the elephants, because these are the parts of the genome that are probably important for making a mammoth a mammoth," Shapiro said. Once we have those, we have to figure out what they mean. How do we map the sequence of As and Cs and Gs and Ts to the way an animal looks and acts? That's one of the hardest problems in biology, to try and match genotypes to phenotypes."
Genome editing
Genetics is itself a relatively new subfield of biology, and gene editing is even newer. Tools like CRISPR (short for clustered regularly interspaced short palindromic repeats) offer gene editing technologies which allows researchers to essentially cut and paste pieces of DNA. Colossal researchers are developing new tools or innovations for existing tools to make editing DNA easier and more efficient.
"Once we have a list [of target genes] we're designing approaches to make hundreds or thousands of changes to a genome simultaneously," Shapiro said. "Our genome engineering team are improving technologies that already exist and developing new technologies to swap out giant chunks of a genome at a time or to make hundreds of different edits all at the same time."
Researchers will take living cells from an Asian elephant and modify them with the desired mammoth traits. Those modified cells will form half of the eventual mammoth embryo. Those newly developed gene editing tools will simplify the process of making a mammoth but they might be more broadly applicable, in the meantime. "As we break these limits, these are tools that have applications outside of de-extinction and conservation, including in human health," Shapiro said.
Embryo creation
Once the gene edits are completed, the modified elephant cell's DNA will be transplanted into the egg cell of an Asian elephant in a process known as somatic cell nuclear transfer. The process involves removing the nucleus of the egg cell, replacing it with the nucleus of the modified donor cell, and activating the new cell. The process is more or less the same as the one which gave us Dolly the cloned sheep, but things get more complicated when it comes to cloning the dodo and other birds.
"For somatic cell nuclear transfer to work, you have to have access to an egg cell that is sort of 'ripe,' ready to be fertilized but not yet fertilized. In birds, this process happens simultaneously. By the time the egg is laid and accessible, it's really difficult," Shapiro said.
Instead, scientists have to get at the bird germ (sperm or egg) cells before the egg is laid, and they do it by going back a generation. When an egg is laid, the embryo is about 24 hours old and the cells that will become germ cells are circulating in the embryo's blood. Researchers suck out that blood, culture those primordial germ cells, modify them, and stick them into another embryo at the same stage of development. The bird that's born isn't modified, but its babies will be.
Embryo implantation
The final step in the process is implanting the embryo into a surrogate womb. Colossal is looking at both Asian and African elephants as the potential surrogate. Asian elephants are more closely related, but African elephants are larger and might be more capable of carrying something mammoth-like to term. In the more distant future, mammoths and other animals might be born in artificial wombs.
"We also have a team called ExoDev, Exogenous Development, that's trying to create an external uterus, where we can have a complete cycle from fertilization to birth, outside of using a surrogate host. That's downstream," Shapiro said.
"None of our first generation species will be born ex-utero," Lamm added. "So many of these things will have long-term applications not just for human healthcare but for conservation. If we're successful in our artificial wombs, that not only allows us to scale quote-unquote production of de-extinction of certain species, but it also allows us to scale conservation efforts. Imagine a world where we can grow 100 northern white rhinos that have engineered genetic diversity in them, all in a laboratory, then work with incredible rewilding partners to put them back in the wild." A world where we can resurrect extinct species is pretty cool, but a world where we slow or stop human-driven extinction is even better.